AAMI Announces 2020 Winners of BI&T Awards

For Immediate Release
This year, AAMI is recognizing sterility assurance researchers, a cybersecurity expert, and a multidisciplinary team of biomedical engineers, IT professionals, clinicians, human factors engineers, and administrators for their outstanding contributions to AAMI’s peer-reviewed journal, BI&T.
These articles are selected each year by the BI&T Editorial Board to be honored at the AAMI Exchange as well as to receive a $1,000 award. Winners are selected for their contributions as the best article, research paper, and commentary published in BI&T in 2020.
“The areas covered by this year’s BI&T award winners are vast, spanning from the management of clinical alarms to the processing of single-use PPE during, and ensuring the cybersecurity of medical devices. But all have a measurable effect on the effectiveness of health technology and the safety of patients who rely on it,” said Gavin Stern, MPH, MS, editor in chief of AAMI publications. “AAMI thanks all of the authors for advancing their fields and for sharing their experience with the health technology community.”
Best Article
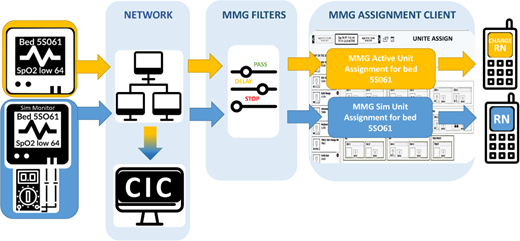
“Protocol for a New Method to Measure Physiologic Monitor Alarm Responsiveness”
(BI&T, November/December 2020)
In this analysis article, the authors (a multidisciplinary team of biomedical engineers, human factors engineers, IT specialists, clinicians, simulation center facilitators, clinical informaticians, and hospital administrators) described a simulation approach to measuring clinical alarm response times and rates—a system that is readily reproducible and uses existing hospital technology.
That data “can be used to design and evaluate quality improvement efforts to address alarm responsiveness and to benchmark performance of different alarm communication systems,” the authors wrote.
Authors:
-
Brooke Luo, MD, clinical instructor in the Departments of Pediatrics and Biomedical Health Informatics at the Children's Hospital of Philadelphia.
-
Melissa McLoone, BSN, RN, nursing practice specialist fellow at the Children's Hospital of Philadelphia
-
Irit R. Rasooly, MD, MSCE, clinical instructor in the Departments of Pediatrics and Biomedical Health Informatics at the Children's Hospital of Philadelphia
-
Sansanee Craig, MD, clinical instructor in the Departments of Pediatrics and Biomedical Health Informatics at the Children's Hospital of Philadelphia in Philadelphia
-
Naveen Muthu, MD, clinical instructor in the Departments of Pediatrics and Biomedical Health Informatics at the Children's Hospital of Philadelphia
-
James Won, PhD, human factors engineer manager and adjunct assistant professor in the Center for Healthcare Quality and Analytics at the Children's Hospital of Philadelphia
-
Halley Ruppel, PhD, RN, staff scientist in the Division of Research at Kaiser Permanente Northern California in Oakland, CA
-
Christopher P. Bonafide, MD, MSCE, an associate professor in the Departments of Pediatrics and Biomedical and Health Informatics at the Children's Hospital of Philadelphia
Best Research Paper
“Dry Heat Processing of Single-Use Respirators and Surgical Masks”
(BI&T, November/December 2020)
Pandemic preparedness plans should include adequate amounts of personal protective equipment (PPE) supplies. But when a public health emergency results in a shortage—as occurred during the COVID-19 pandemic—it may be necessary to decontaminate and reuse supplies that were intended to be used only once, such as respirators and surgical masks. In this research article, a team of researchers at Johnson & Johnson demonstrated dry heat processing as an “appropriate method” for repeated single-use respirator and surgical mask decontamination.
The authors elected to donate their $1,000 award to the Kilmer Scholarship Fund.
Authors:
- Joyce M. Hansen, BS, MBA, vice president of microbiological quality & sterility assurance at Johnson & Johnson
- Scott Weiss, BS, director of industrial microbiology at Johnson & Johnson
- Terra A. Kremer, BS, senior program manager in Microbiological Quality & Sterility Assurance at Johnson & Johnson
- Myrelis Aguilar, BS, senior staff scientist at Johnson & Johnson in Raritan, NJ
- Gerald McDonnell, BSc, PhD, senior director in Microbiological Quality & Sterility Assurance at Johnson & Johnson
Best Commentary

“Talking about the Software Supply Chain”
(BI&T, September/October 2020)
What defines your software supply chain is highly dependent of your point of view, as a healthcare delivery organization or a medical device manufacturer. But as cybersecurity expert Axel Wirth explains in his “CyberInsights” column, understanding the full extent of all the ingredients in the final product you buy (as an HDO) or ship (as an MDM or supplier) is a recognized necessity and prerequisite for understanding a device's security posture, as well as for managing and maintaining it in the field.
Author:
Axel Wirth, CPHIMS, CISSP, HCISPP, AAMIF, FHIMSS, chief security strategist at MedCrypt in San Diego, CA.
For more information on BI&T, visit www.aami.org/BIT.
About AAMI
AAMI (www.aami.org) is a nonprofit organization founded in 1967. It is a diverse community of more than 9,000 healthcare technology professionals united by one important mission—supporting the healthcare community in the development, management, and use of safe and effective health technology. AAMI is the primary source of consensus standards, both national and international, for the medical device industry, as well as practical information, support, and guidance for health technology and sterilization professionals.
Contacts




